Strategy For Regulatory Compliance Mdr Template - Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. In this document, you will find a list of medqdoc’s templates for mdr technical documentation, that can be used to ensure your medical device. Covering iso 13485, iec 62304, iso 14971 and iec 62366.
Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. In this document, you will find a list of medqdoc’s templates for mdr technical documentation, that can be used to ensure your medical device. Covering iso 13485, iec 62304, iso 14971 and iec 62366.
Covering iso 13485, iec 62304, iso 14971 and iec 62366. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. In this document, you will find a list of medqdoc’s templates for mdr technical documentation, that can be used to ensure your medical device.
Regulatory Strategy Template
In this document, you will find a list of medqdoc’s templates for mdr technical documentation, that can be used to ensure your medical device. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. Covering iso 13485, iec 62304, iso 14971 and iec 62366.
Regulatory Strategy Template
In this document, you will find a list of medqdoc’s templates for mdr technical documentation, that can be used to ensure your medical device. Covering iso 13485, iec 62304, iso 14971 and iec 62366. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices.
Strategy for Regulatory Compliance according to EU MDR 2017/745
Covering iso 13485, iec 62304, iso 14971 and iec 62366. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. In this document, you will find a list of medqdoc’s templates for mdr technical documentation, that can be used to ensure your medical device.
Regulatory Strategy Template prntbl.concejomunicipaldechinu.gov.co
Covering iso 13485, iec 62304, iso 14971 and iec 62366. In this document, you will find a list of medqdoc’s templates for mdr technical documentation, that can be used to ensure your medical device. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices.
The Essential Guide to Preparing your QMS for EU MDR
Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. In this document, you will find a list of medqdoc’s templates for mdr technical documentation, that can be used to ensure your medical device. Covering iso 13485, iec 62304, iso 14971 and iec 62366.
EU MDR Compliance and EU MDR Implementation Strategy Services Regulatory Compliance Associates
Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. In this document, you will find a list of medqdoc’s templates for mdr technical documentation, that can be used to ensure your medical device. Covering iso 13485, iec 62304, iso 14971 and iec 62366.
Strategy For Regulatory Compliance Mdr Template
Covering iso 13485, iec 62304, iso 14971 and iec 62366. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. In this document, you will find a list of medqdoc’s templates for mdr technical documentation, that can be used to ensure your medical device.
FREE Compliance Templates & Examples Edit Online & Download
Covering iso 13485, iec 62304, iso 14971 and iec 62366. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. In this document, you will find a list of medqdoc’s templates for mdr technical documentation, that can be used to ensure your medical device.
Regulatory Strategy Template
Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices. In this document, you will find a list of medqdoc’s templates for mdr technical documentation, that can be used to ensure your medical device. Covering iso 13485, iec 62304, iso 14971 and iec 62366.
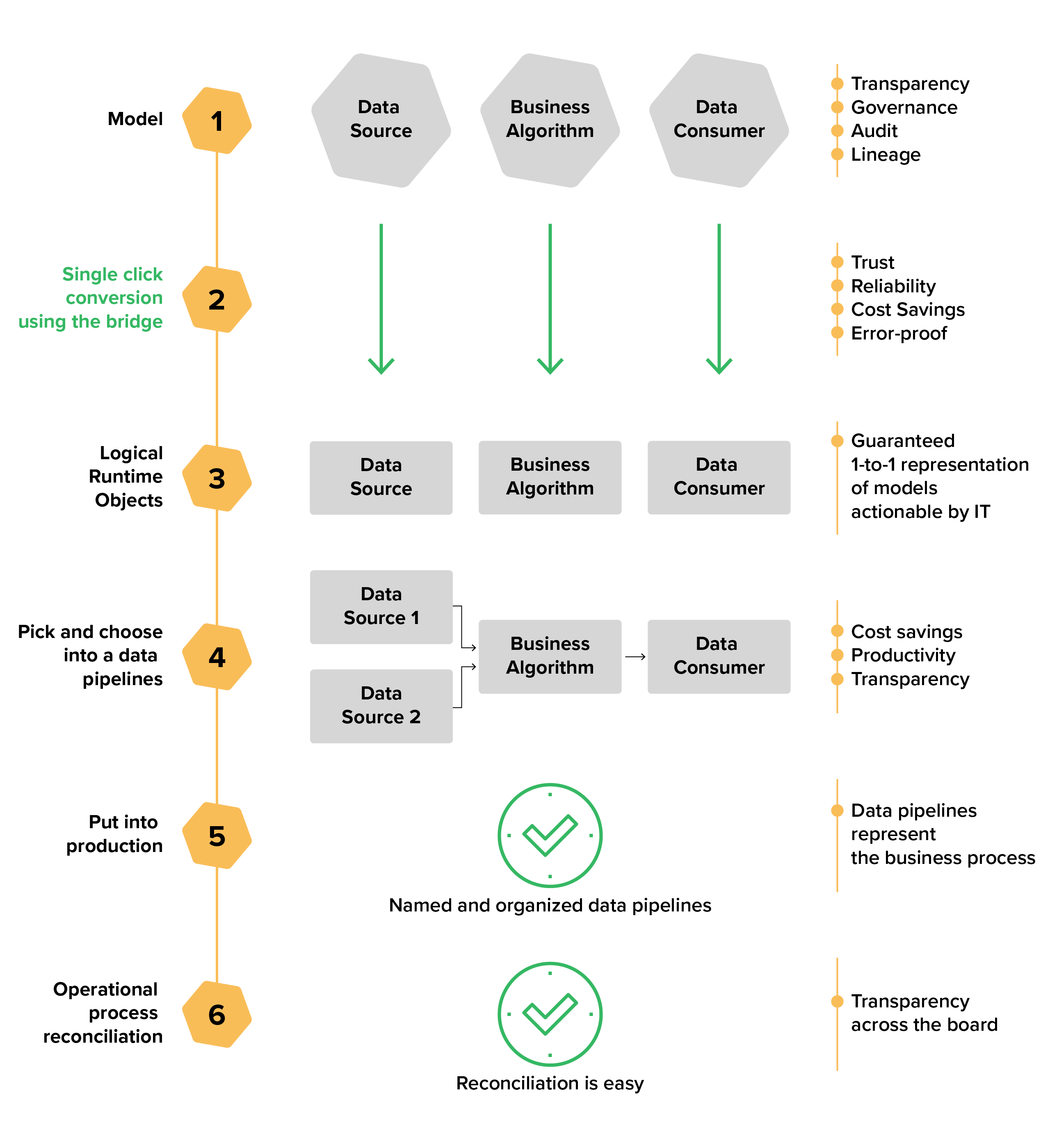
MDR Compliance Gap Analysis Spreadsheet [format *.xlsx] M.L. Reo Consulting
In this document, you will find a list of medqdoc’s templates for mdr technical documentation, that can be used to ensure your medical device. Covering iso 13485, iec 62304, iso 14971 and iec 62366. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices.
Covering Iso 13485, Iec 62304, Iso 14971 And Iec 62366.
In this document, you will find a list of medqdoc’s templates for mdr technical documentation, that can be used to ensure your medical device. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 (ivdr) on in vitro diagnostic medical devices.








![MDR Compliance Gap Analysis Spreadsheet [format *.xlsx] M.L. Reo Consulting](https://mlreoconsulting.com/wp-content/uploads/2022/11/MDR-Gap-Analysis.jpg)