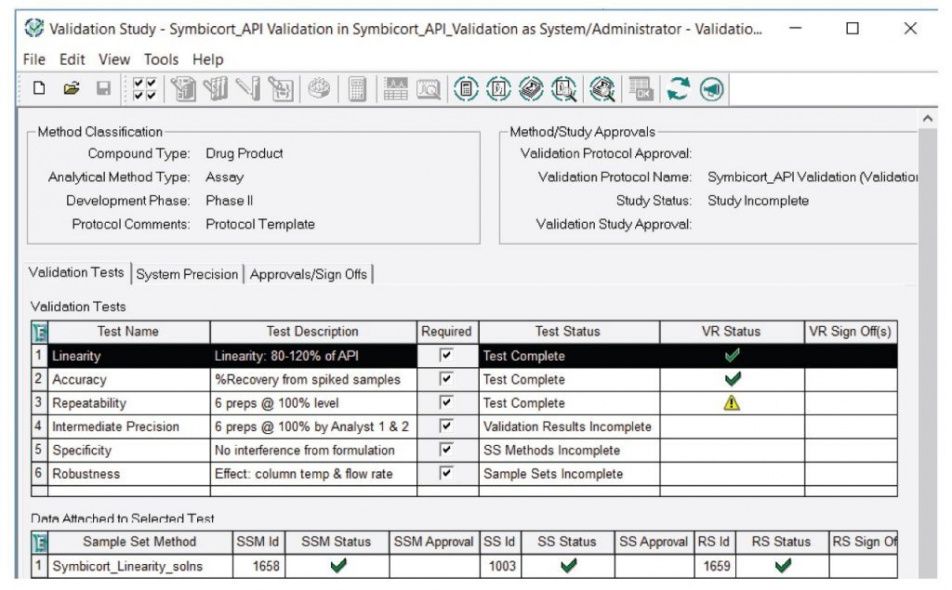
Medical Device Verification And Validation Plan Template - Process verification and process validation are two important—and commonly misunderstood—activities in the development. Please note that every device is different and. The second half of the guidance. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory. Validation confirms that the system meets the user needs and intended use. Presuming a medical device product, here's a general outline of what we typically use. What do you do to demonstrate that?
Validation confirms that the system meets the user needs and intended use. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory. What do you do to demonstrate that? The second half of the guidance. Process verification and process validation are two important—and commonly misunderstood—activities in the development. Please note that every device is different and. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Presuming a medical device product, here's a general outline of what we typically use.
Please note that every device is different and. Process verification and process validation are two important—and commonly misunderstood—activities in the development. What do you do to demonstrate that? A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. The second half of the guidance. Presuming a medical device product, here's a general outline of what we typically use. Validation confirms that the system meets the user needs and intended use. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory.
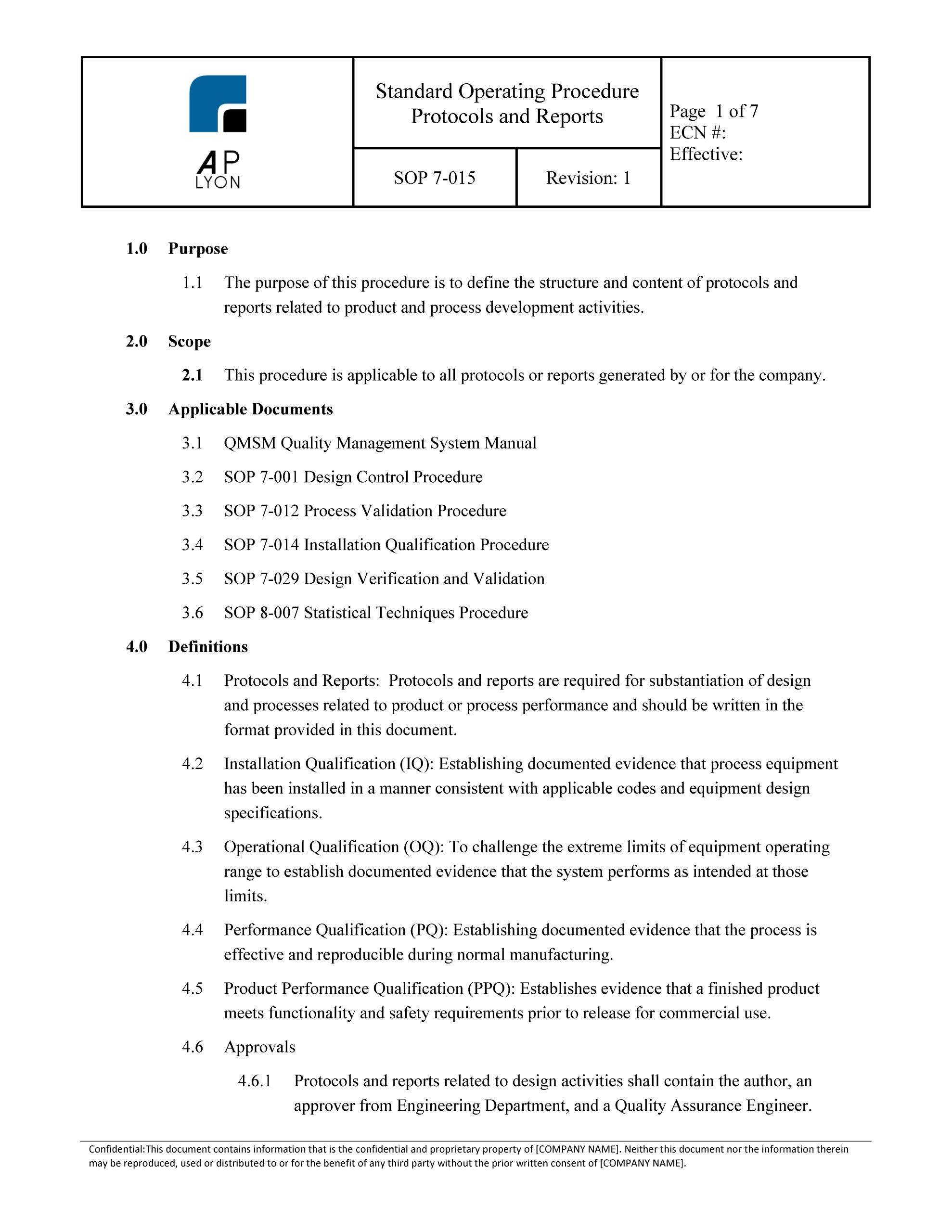
Medical Device Verification And Validation Plan Template
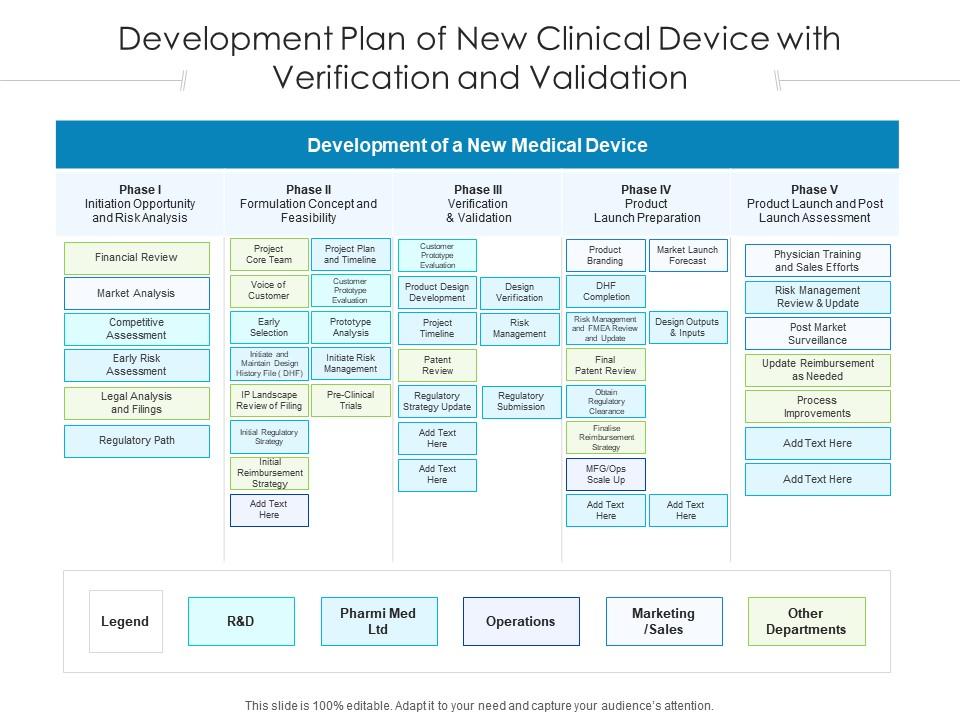
Presuming a medical device product, here's a general outline of what we typically use. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory. Process verification and process.
Medical Device Verification And Validation Plan Template prntbl
Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory. Process verification and process validation are two important—and commonly misunderstood—activities in the development. Validation confirms that the system meets the user needs and intended use. The second half of the guidance. A free master validation plan (mvp) form to help.
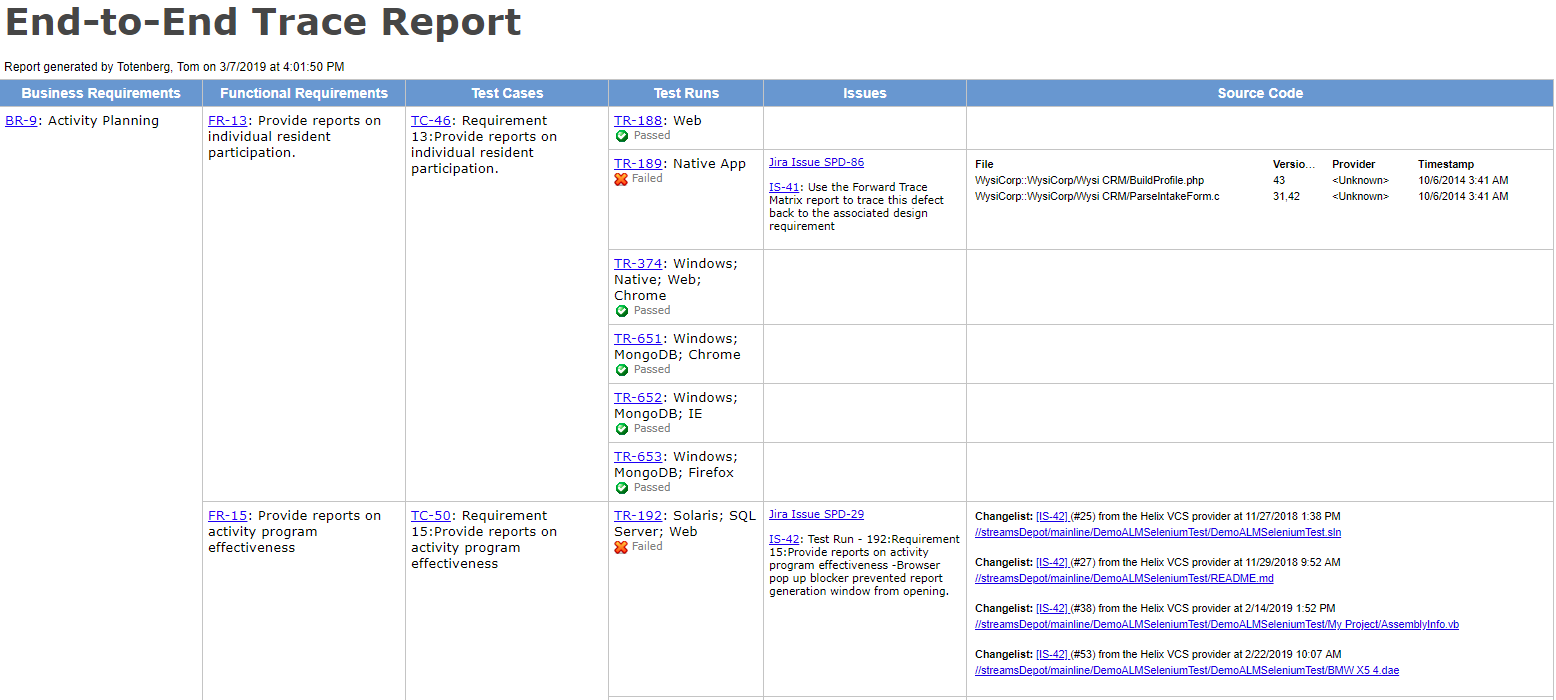
Free Medical Device Validation Report Template Word Example Stableshvf
Please note that every device is different and. Validation confirms that the system meets the user needs and intended use. Presuming a medical device product, here's a general outline of what we typically use. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Process verification and process.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Process verification and process validation are two important—and commonly misunderstood—activities in the development. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. What do you do to demonstrate that? The second half of the guidance. Medqdoc provides 28 templates to support you in compiling the correct technical.
Verification and Validation Plan Template (MS Word) Templates, Forms
A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory. Presuming a medical device product, here's a general outline of what we typically use. The second half of.
Medical Device Verification And Validation Plan Template
Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory. Presuming a medical device product, here's a general outline of what we typically use. Validation confirms that the system meets the user needs and intended use. The second half of the guidance. Please note that every device is different and.
Medical Device Verification And Validation Plan Template
What do you do to demonstrate that? Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory. Process verification and process validation are two important—and commonly misunderstood—activities in the development. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes.
Medical Device Verification And Validation Plan Template prntbl
Please note that every device is different and. Validation confirms that the system meets the user needs and intended use. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. The second half of the guidance. Medqdoc provides 28 templates to support you in compiling the correct technical.
Medical Device Verification And Validation Plan Template prntbl
Validation confirms that the system meets the user needs and intended use. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory. The second half of the guidance..
Free Medical Device Validation Report Template Excel Sample Stableshvf
Validation confirms that the system meets the user needs and intended use. What do you do to demonstrate that? Please note that every device is different and. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory. Process verification and process validation are two important—and commonly misunderstood—activities in the development.
Please Note That Every Device Is Different And.
What do you do to demonstrate that? Validation confirms that the system meets the user needs and intended use. Presuming a medical device product, here's a general outline of what we typically use. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory.
The Second Half Of The Guidance.
Process verification and process validation are two important—and commonly misunderstood—activities in the development. A free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation.