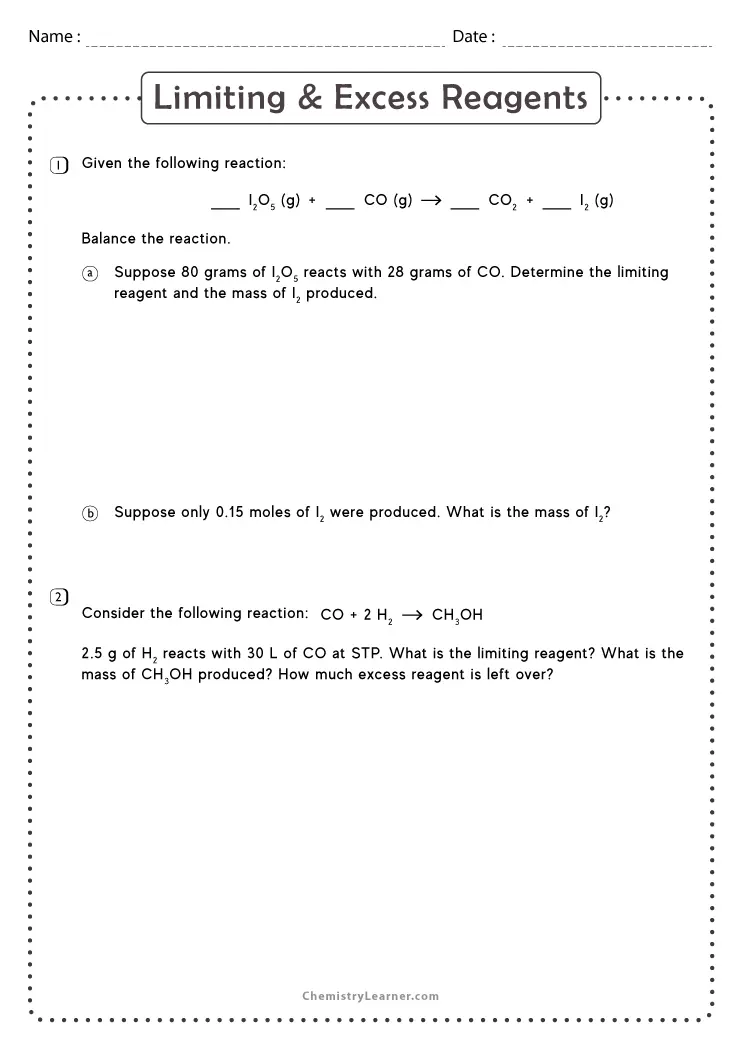
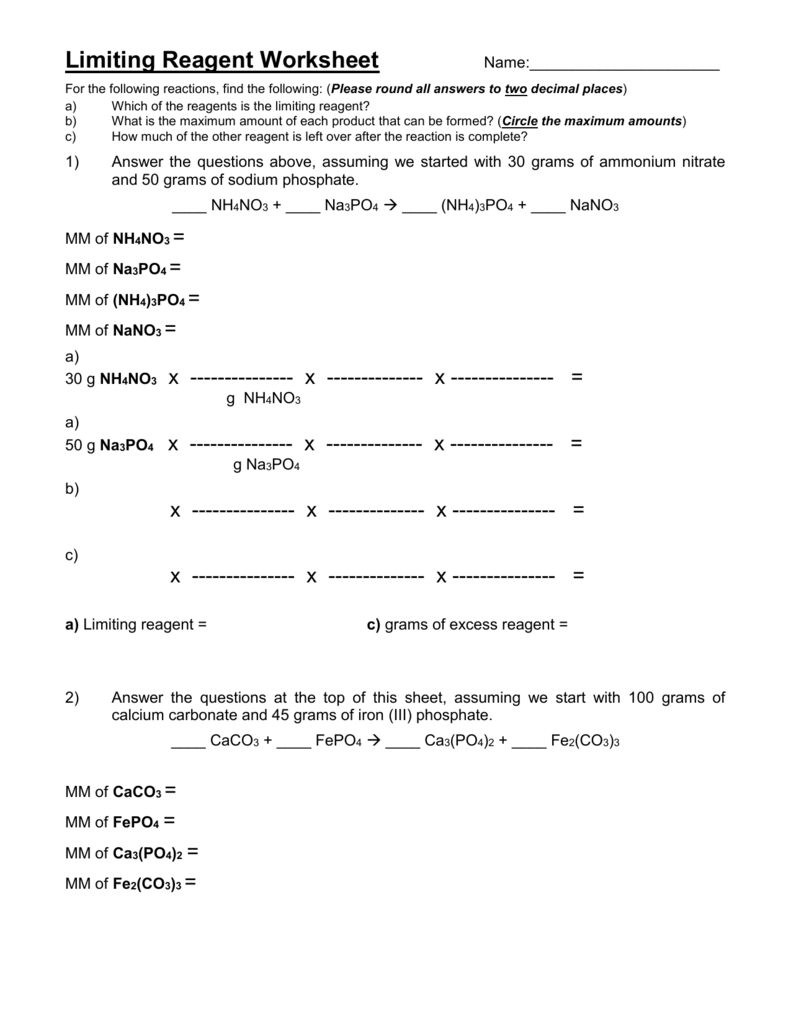
Limiting Reagent Worksheet 1 Answers - Can use either of the following to determine the limiting reactant. Limiting reagent worksheet #1 1. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? What is the limiting reactant? Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Determine the number of grams of excess reagent left over in the reaction. (balance the equation first!) +5 02 3 c02 + h20 a) if you start with 14.8 g of. If 25.4 g of al2o3 is reacted with 10.2 g of. Because sodium iodide is the reagent that causes 8.51 grams of sodium. What is the limiting reagent in the reaction described in problem 2?
What is the limiting reagent in the reaction described in problem 2? Because sodium iodide is the reagent that causes 8.51 grams of sodium. If 25.4 g of al2o3 is reacted with 10.2 g of. Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Determine the number of grams of excess reagent left over in the reaction. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Limiting reagent worksheet #1 1. What is the limiting reactant? Can use either of the following to determine the limiting reactant. (balance the equation first!) +5 02 3 c02 + h20 a) if you start with 14.8 g of.
Because sodium iodide is the reagent that causes 8.51 grams of sodium. If 25.4 g of al2o3 is reacted with 10.2 g of. Limiting reagent worksheet #1 1. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? (balance the equation first!) +5 02 3 c02 + h20 a) if you start with 14.8 g of. What is the limiting reactant? Can use either of the following to determine the limiting reactant. What is the limiting reagent in the reaction described in problem 2? Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Determine how much one of the reactant needs.
Limiting Reagent And Percent Yield Worksheet
If 25.4 g of al2o3 is reacted with 10.2 g of. What is the limiting reactant? Because sodium iodide is the reagent that causes 8.51 grams of sodium. (balance the equation first!) +5 02 3 c02 + h20 a) if you start with 14.8 g of. How many moles of nh3 can be produced from the reaction of 28 g.
Limiting Reagent Worksheet
If 25.4 g of al2o3 is reacted with 10.2 g of. Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Can use either of the following to determine the limiting reactant. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Because.
Limiting Reagent Problems And Answers
Can use either of the following to determine the limiting reactant. What is the limiting reagent in the reaction described in problem 2? Limiting reagent worksheet #1 1. Because sodium iodide is the reagent that causes 8.51 grams of sodium. What is the limiting reactant?
Free Printable Limiting Reagent Worksheets
How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Because sodium iodide is the reagent that causes 8.51 grams of sodium. (balance the equation first!) +5 02 3 c02 + h20 a) if you start with 14.8 g of. Determine how much one of the reactant needs. What is the limiting reactant?
Limiting Reagent Worksheets 1 Answers
If 25.4 g of al2o3 is reacted with 10.2 g of. (balance the equation first!) +5 02 3 c02 + h20 a) if you start with 14.8 g of. What is the limiting reactant? Determine how much one of the reactant needs. Because sodium iodide is the reagent that causes 8.51 grams of sodium.
Limiting Reagent Worksheet 1 Pdf —
Can use either of the following to determine the limiting reactant. Determine how much one of the reactant needs. Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Limiting reagent worksheet #1 1. How many moles of nh3 can be produced from the reaction of 28 g of n2.
Limiting Reagent Worksheet 1
If 25.4 g of al2o3 is reacted with 10.2 g of. (balance the equation first!) +5 02 3 c02 + h20 a) if you start with 14.8 g of. Can use either of the following to determine the limiting reactant. Because sodium iodide is the reagent that causes 8.51 grams of sodium. Determine how much one of the reactant needs.
limiting reagent worksheet answers.notebook
Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. What is the limiting reagent in the reaction described in problem 2? Can use either of the following to determine the limiting reactant. Because sodium iodide is the reagent that causes 8.51 grams of sodium. What is the limiting reactant?
Limiting Reagent Practice Problems With Answers Limiting Rea
Determine how much one of the reactant needs. If 25.4 g of al2o3 is reacted with 10.2 g of. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? What is the limiting reactant? What is the limiting reagent in the reaction described in problem 2?
Limiting Reagent Problems And Answers Limiting Reagent (reac
How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Determine the number of grams of excess reagent left over in the reaction. What is the limiting reactant? Can use either of the following to determine the limiting reactant. If 25.4 g of al2o3 is reacted with 10.2 g of.
Because Sodium Iodide Is The Reagent That Causes 8.51 Grams Of Sodium.
Can use either of the following to determine the limiting reactant. If 25.4 g of al2o3 is reacted with 10.2 g of. (balance the equation first!) +5 02 3 c02 + h20 a) if you start with 14.8 g of. What is the limiting reagent in the reaction described in problem 2?
How Many Moles Of Nh3 Can Be Produced From The Reaction Of 28 G Of N2 ?
Determine how much one of the reactant needs. What is the limiting reactant? Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Determine the number of grams of excess reagent left over in the reaction.