Ideal Gas Law Worksheet With Answers - • some answers provided at the end of the question. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? 10 ideal gas law 1. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the ideal gas. Use your knowledge of the ideal and combined gas laws to solve the following problems. The ideal gas law directions: Solve each of the following problems. • the column on the right is so you can practice quickly identifying. Show your work, including proper units, to earn full credit. Pv = nrt solving for p.
Apply the ideal gas law: Show your work, including proper units, to earn full credit. Solve each of the following problems. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the ideal gas. • the column on the right is so you can practice quickly identifying. Use your knowledge of the ideal and combined gas laws to solve the following problems. Pv = nrt solving for p. • some answers provided at the end of the question. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? The ideal gas law directions:
10 ideal gas law 1. Use your knowledge of the ideal and combined gas laws to solve the following problems. • some answers provided at the end of the question. Depending which r value you use, tells you which label to put on your resulting pressure value. Apply the ideal gas law: The ideal gas law directions: Solve each of the following problems. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the ideal gas. Pv = nrt solving for p. If it involves moles or grams, it must be pv = nrt.
Chemistry Ideal Gas Law Worksheet
• some answers provided at the end of the question. Show your work, including proper units, to earn full credit. The ideal gas law directions: Pv = nrt solving for p. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of.
Gas Laws Ideal Gas Law Worksheet Ideal Gas Law Worksheet Ans
The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the ideal gas. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Show your work, including.
Chemistry Gas Laws Worksheet
Use your knowledge of the ideal and combined gas laws to solve the following problems. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? The ideal gas law directions: Pv = nrt solving for p. Depending which r value you use, tells you which label to put on your.
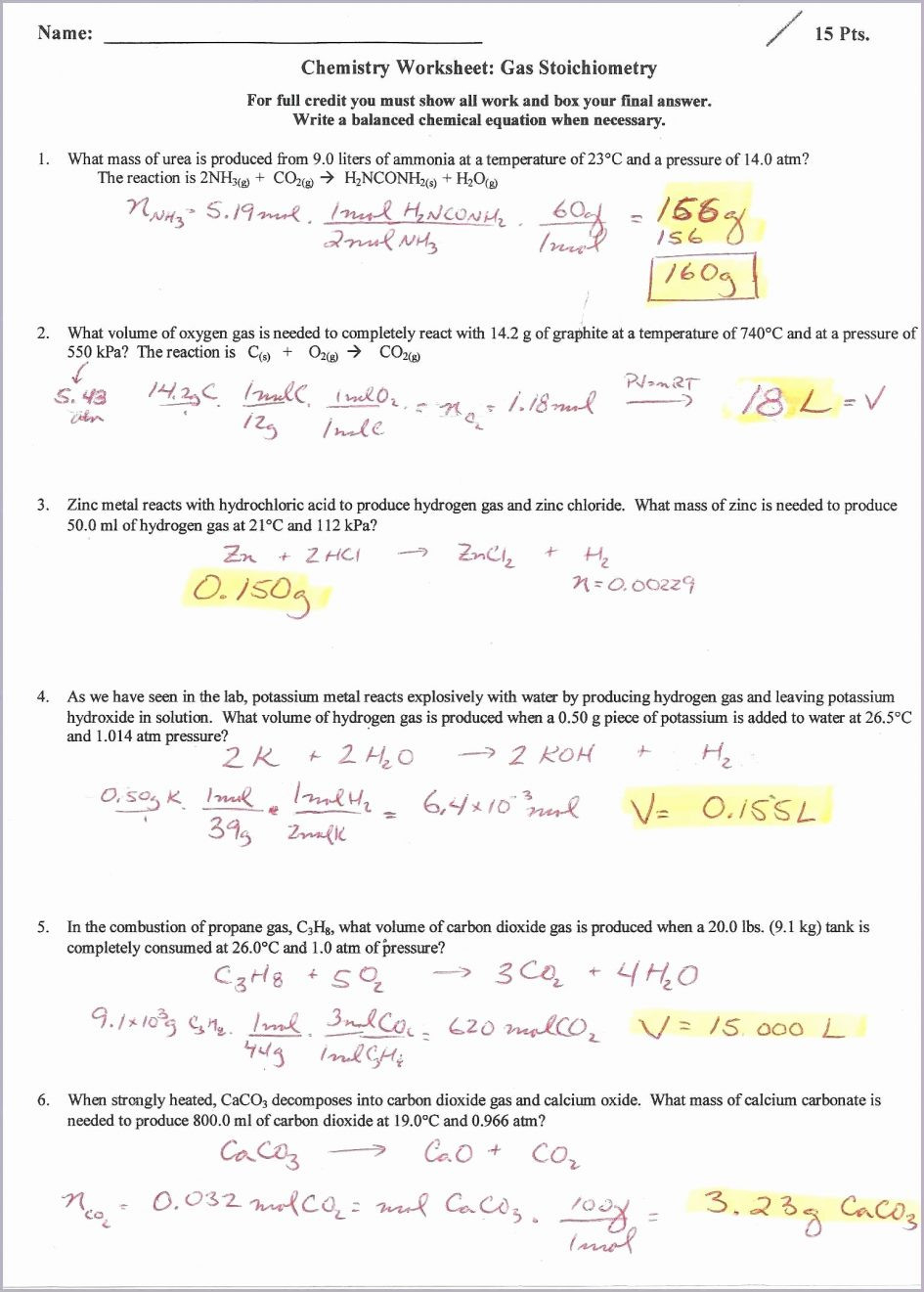
Worksheet Gas Stoichiometry Worksheet Ideal Gas Law —
The ideal gas law directions: How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? • some answers provided at the end of the question. Apply the ideal gas law: Use your knowledge of the ideal and combined gas laws to solve the following problems.
Ideal Gas Law Chem Worksheet 144 Answer Key LINK
Solve each of the following problems. Use your knowledge of the ideal and combined gas laws to solve the following problems. Pv = nrt solving for p. Depending which r value you use, tells you which label to put on your resulting pressure value. 10 ideal gas law 1.
Quiz & Worksheet Ideal Gas Law and the Gas Constant
Depending which r value you use, tells you which label to put on your resulting pressure value. 10 ideal gas law 1. The ideal gas law directions: • some answers provided at the end of the question. Solve each of the following problems.
Gas Law Stoichiometry Worksheet Answer
If it involves moles or grams, it must be pv = nrt. • some answers provided at the end of the question. • the column on the right is so you can practice quickly identifying. Use your knowledge of the ideal and combined gas laws to solve the following problems. Pv = nrt solving for p.
Chemistry The Ideal Gas Law Worksheet Answers. Web for our experimental
Pv = nrt solving for p. Apply the ideal gas law: The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the ideal gas. • some answers provided at the end of the question. Use your knowledge.
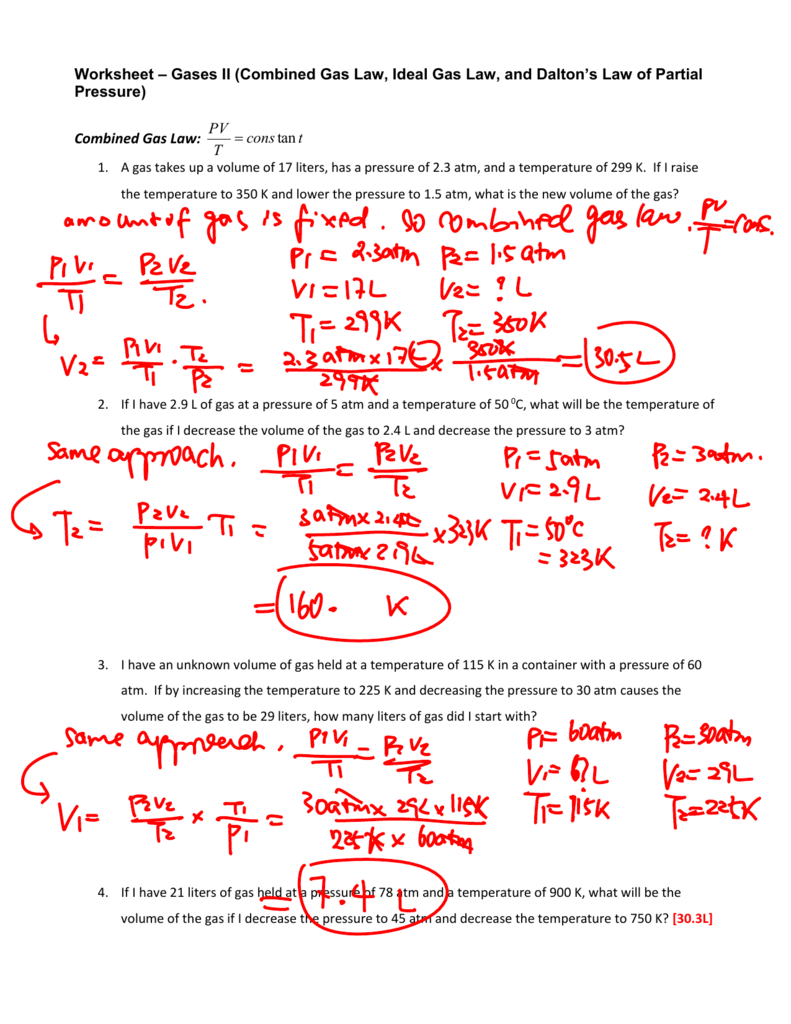
Worksheet Combined Gas Law And Ideal Gas Law
Use your knowledge of the ideal and combined gas laws to solve the following problems. 10 ideal gas law 1. • some answers provided at the end of the question. Show your work, including proper units, to earn full credit. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l?
Combined Gas Law And Ideal Gas Law Worksheet Combined Gas La
Apply the ideal gas law: Pv = nrt solving for p. • the column on the right is so you can practice quickly identifying. Use your knowledge of the ideal and combined gas laws to solve the following problems. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the.
10 Ideal Gas Law 1.
If it involves moles or grams, it must be pv = nrt. • the column on the right is so you can practice quickly identifying. Pv = nrt solving for p. Use your knowledge of the ideal and combined gas laws to solve the following problems.
Depending Which R Value You Use, Tells You Which Label To Put On Your Resulting Pressure Value.
Solve each of the following problems. Show your work, including proper units, to earn full credit. Apply the ideal gas law: The ideal gas law directions:
The Ideal Gas Law States That Pv=Nrt, Where P Is The Pressure Of A Gas, V Is The Volume Of The Gas, N Is The Number Of Moles Of Gas Present, R Is The Ideal Gas.
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? • some answers provided at the end of the question.