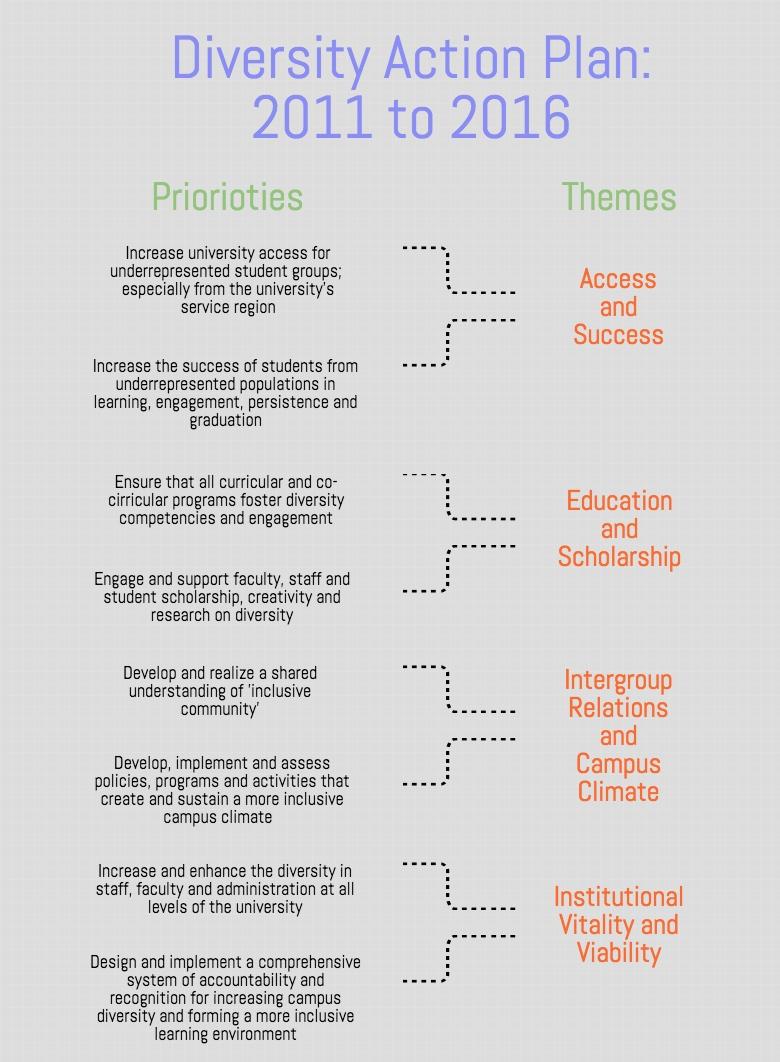
Fda Diversity Plan Template - Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this guidance to. Refer product specific questions regarding diversity. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical studies for which a. Food and drug administration issued a draft guidance, “ diversity action plans to improve enrollment of participants from underrepresented. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. On this page you will find all diversity action plans summary reports for fda.
On this page you will find all diversity action plans summary reports for fda. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical studies for which a. Refer product specific questions regarding diversity. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this guidance to. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. Food and drug administration issued a draft guidance, “ diversity action plans to improve enrollment of participants from underrepresented.
Food and drug administration issued a draft guidance, “ diversity action plans to improve enrollment of participants from underrepresented. Refer product specific questions regarding diversity. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical studies for which a. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this guidance to. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. On this page you will find all diversity action plans summary reports for fda.
Fda Diversity Plan Template
On this page you will find all diversity action plans summary reports for fda. Food and drug administration issued a draft guidance, “ diversity action plans to improve enrollment of participants from underrepresented. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. Refer product specific questions regarding diversity. This draft.
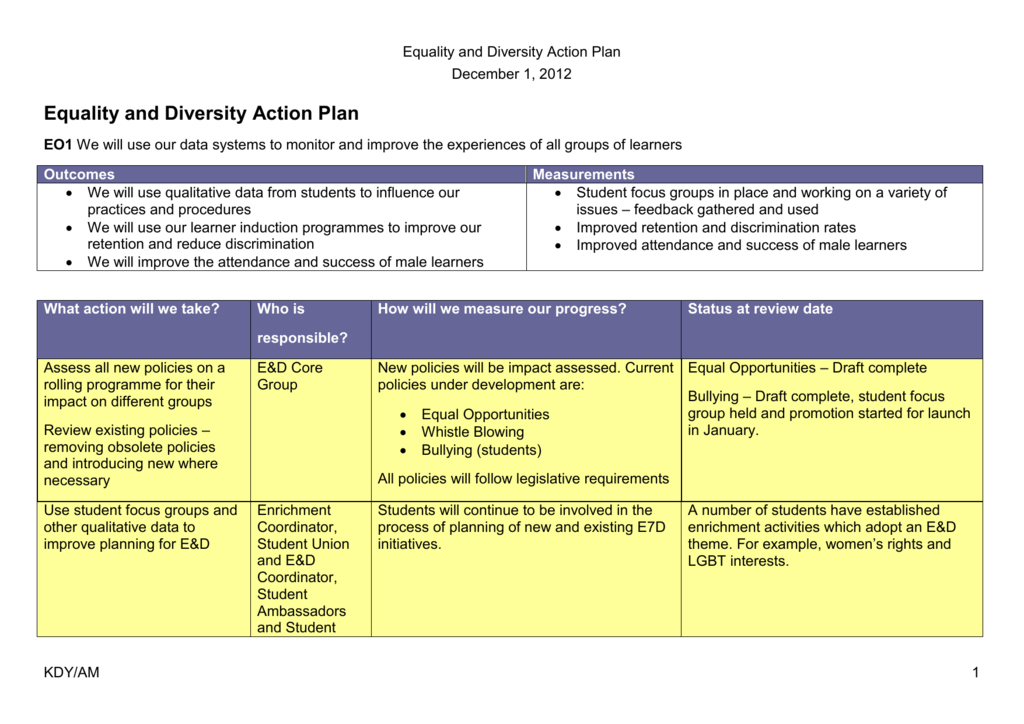
Diversity Equity And Inclusion Plan Template
This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. Food and drug administration issued a draft guidance, “ diversity action plans to improve enrollment of participants from underrepresented. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this guidance to. On this page.
Diversity And Inclusion Action Plan Template prntbl
Refer product specific questions regarding diversity. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this guidance to. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. Food and drug administration issued a draft guidance, “ diversity action plans to improve enrollment of.
Fda Diversity Plan Template
This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical studies for which a. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this guidance.
Fda Diversity Plan Template
Food and drug administration issued a draft guidance, “ diversity action plans to improve enrollment of participants from underrepresented. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this guidance to. Refer product specific questions regarding diversity. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical.
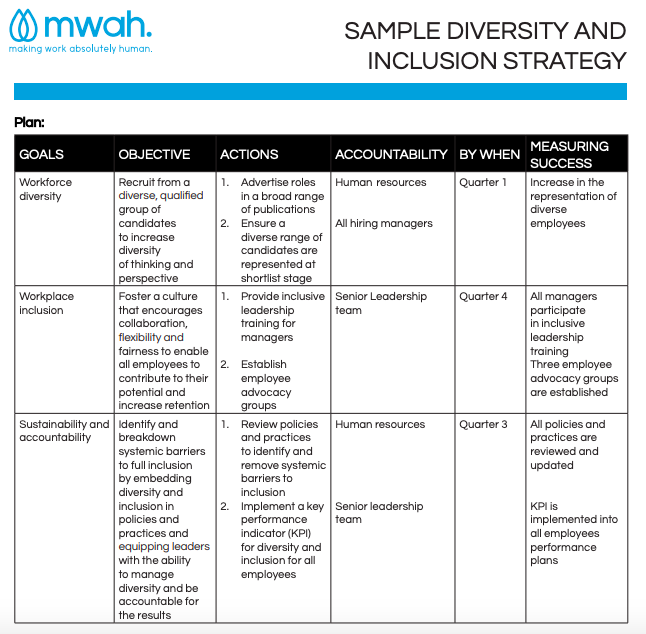
Free Diversity And Inclusion Strategic Plan Template
This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical studies for which a. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this guidance to. On this page you will find all diversity action plans summary reports for fda. Food and drug administration.
Top 10 Diversity and Inclusion Action Plan Templates with Examples and
On this page you will find all diversity action plans summary reports for fda. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this guidance to. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical studies for which a. This draft guidance describes.
Fda Diversity Plan Template
This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical studies for which a. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. On this page you will find all diversity action plans summary reports for fda. Given the importance of.
Fda Diversity Plan Template
On this page you will find all diversity action plans summary reports for fda. Refer product specific questions regarding diversity. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this guidance to. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical studies for.
Fda Diversity Plan Template
Food and drug administration issued a draft guidance, “ diversity action plans to improve enrollment of participants from underrepresented. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this guidance to. This draft guidance.
This Draft Guidance Describes The Form, Content, And Manner Of Diversity Action Plans, The Applicable Medical Products, And Clinical.
On this page you will find all diversity action plans summary reports for fda. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this guidance to. Refer product specific questions regarding diversity. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical studies for which a.