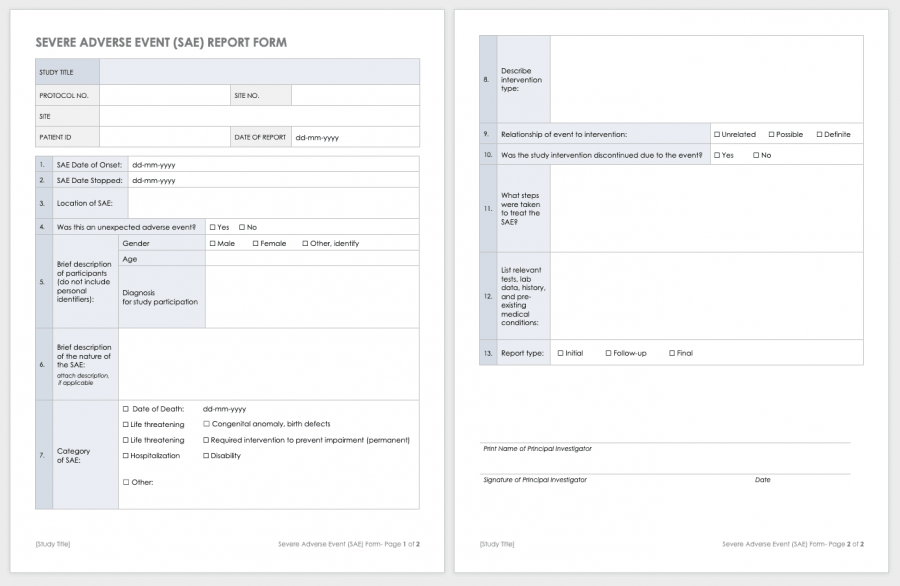
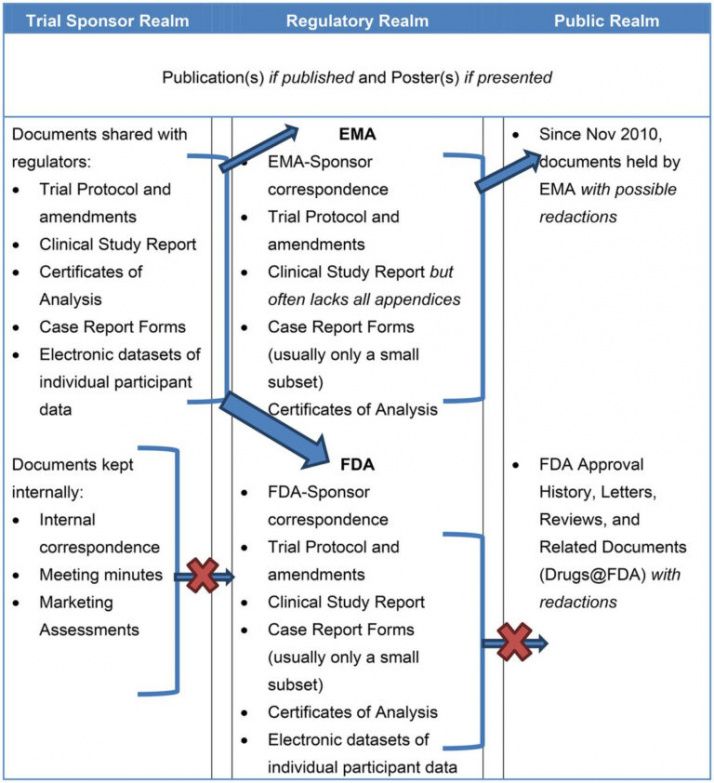
Clinical Study Report Template - This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Learn how to write a data and safety. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. Find protocol, data management, and monitoring templates for clinical trials funded by niams. It covers topics such as.
This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Learn how to write a data and safety. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. It covers topics such as. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of. Find protocol, data management, and monitoring templates for clinical trials funded by niams.
Learn how to write a data and safety. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Find protocol, data management, and monitoring templates for clinical trials funded by niams. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. It covers topics such as.
Clinical Research Report Synopsis Templates At for Clinical Trial
Learn how to write a data and safety. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of. This document provides a note for guidance on the structure.
Clinical Study Report Cardiovascular Diseases Coronary Artery Disease
It covers topics such as. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. Find protocol, data management, and monitoring templates for clinical trials funded by niams. This document.
(PDF) Clinical Study Report (CSR) Clinical Study Report (CSR)
Learn how to write a data and safety. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of. Find protocol, data management, and monitoring templates for clinical trials funded by niams. This document aims.
Free Clinical Trial Templates Smartsheet
This document provides recommendations for the structure and content of clinical study reports submitted to the fda. Learn how to write a data and safety. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports.
Free Clinical Trial Templates Smartsheet
Find protocol, data management, and monitoring templates for clinical trials funded by niams. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. It covers topics such as. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose.
Structure AND Content OF Clinical Study Reports STRUCTURE AND CONTENT
This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. Find protocol, data management, and monitoring templates for clinical trials funded by niams. This document provides.
Clinical Study Report Template PDF Sample Stableshvf
Find protocol, data management, and monitoring templates for clinical trials funded by niams. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. It covers topics such as. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of..
Clinical Study Report (CSR) Template Clinical Study Templates
This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. This document provides.
Clinical Trial Report Template TEMPLATES EXAMPLE TEMPLATES EXAMPLE
This document provides recommendations for the structure and content of clinical study reports submitted to the fda. Find protocol, data management, and monitoring templates for clinical trials funded by niams. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. This document aims to allow the compilation of a.
Clinical Study Report PDF Cardiovascular Diseases Coronary Artery
It covers topics such as. This document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. Find protocol, data management, and monitoring templates for clinical trials funded by niams..
This Document Aims To Allow The Compilation Of A Single Core Clinical Study Report Acceptable To All Regulatory Authorities Of The Ich Regions.
This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. Learn how to write a data and safety. It covers topics such as.
This Document Provides Recommendations For The Structure And Content Of Clinical Study Reports Submitted To The Fda.
Find protocol, data management, and monitoring templates for clinical trials funded by niams. This document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of.