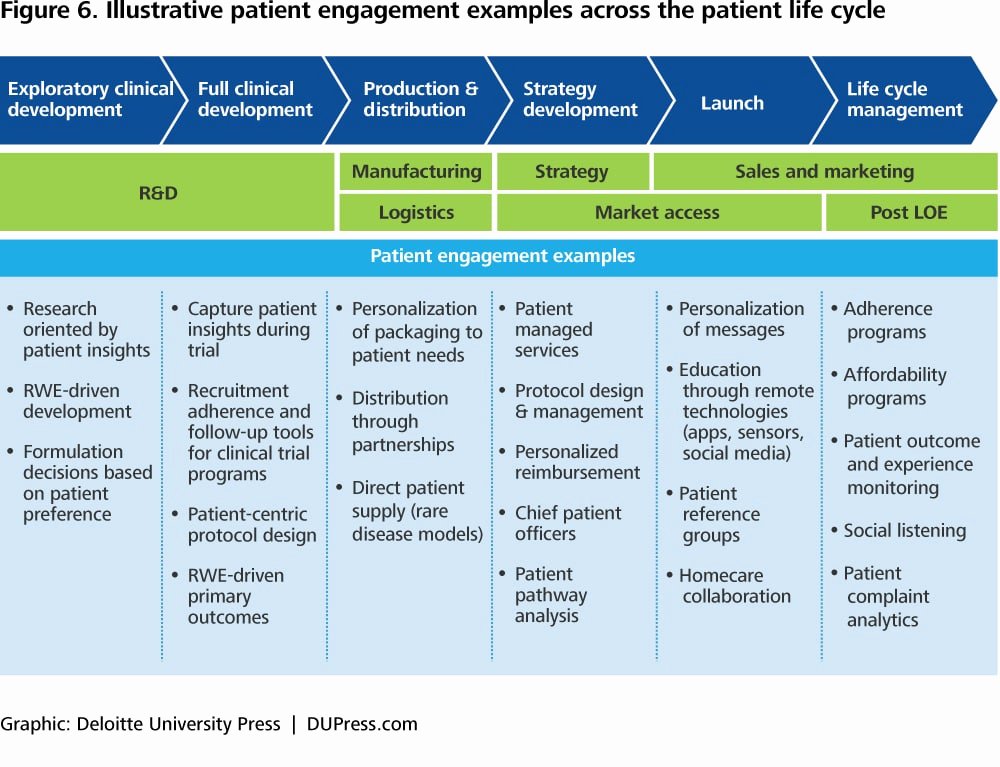
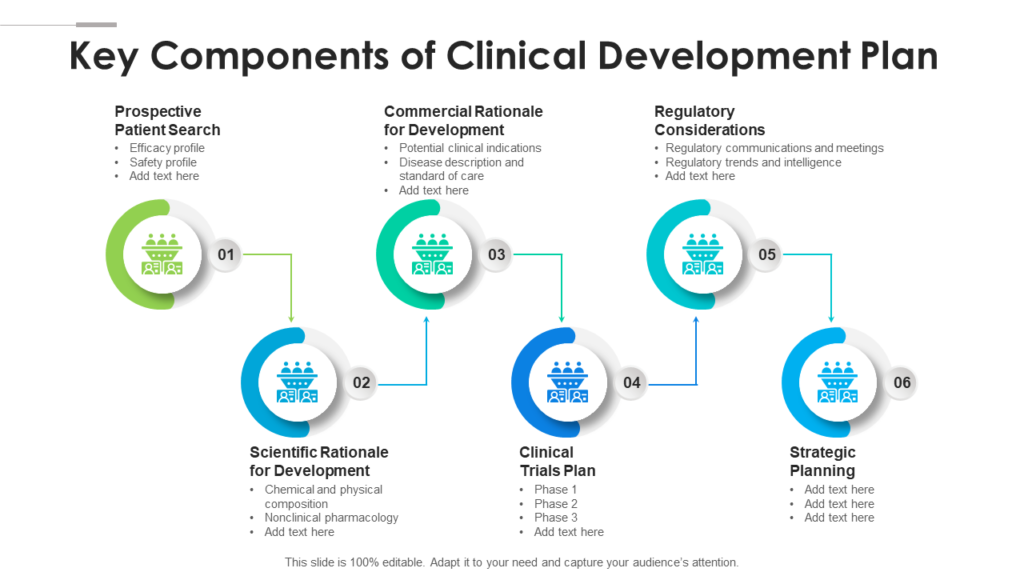
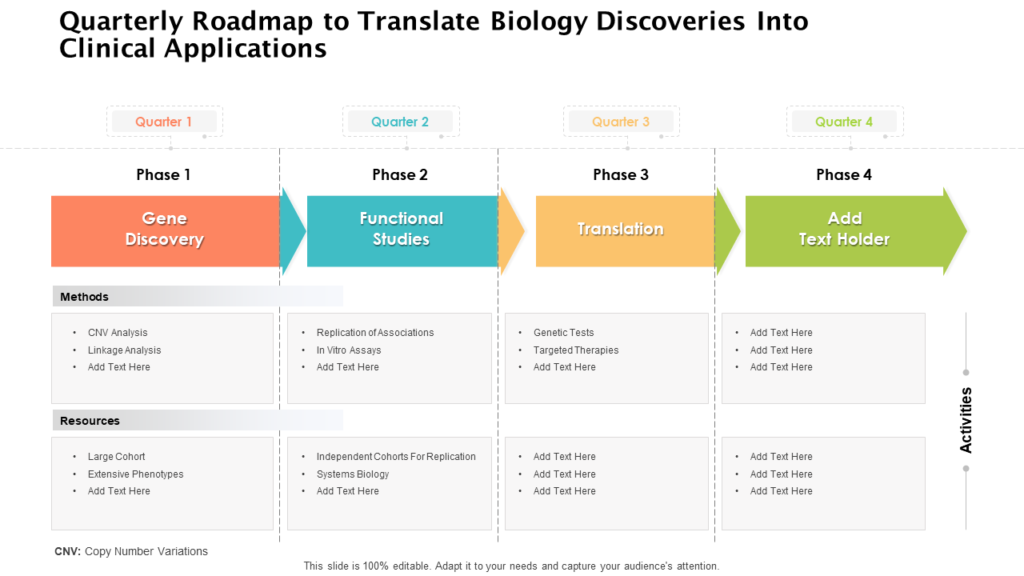
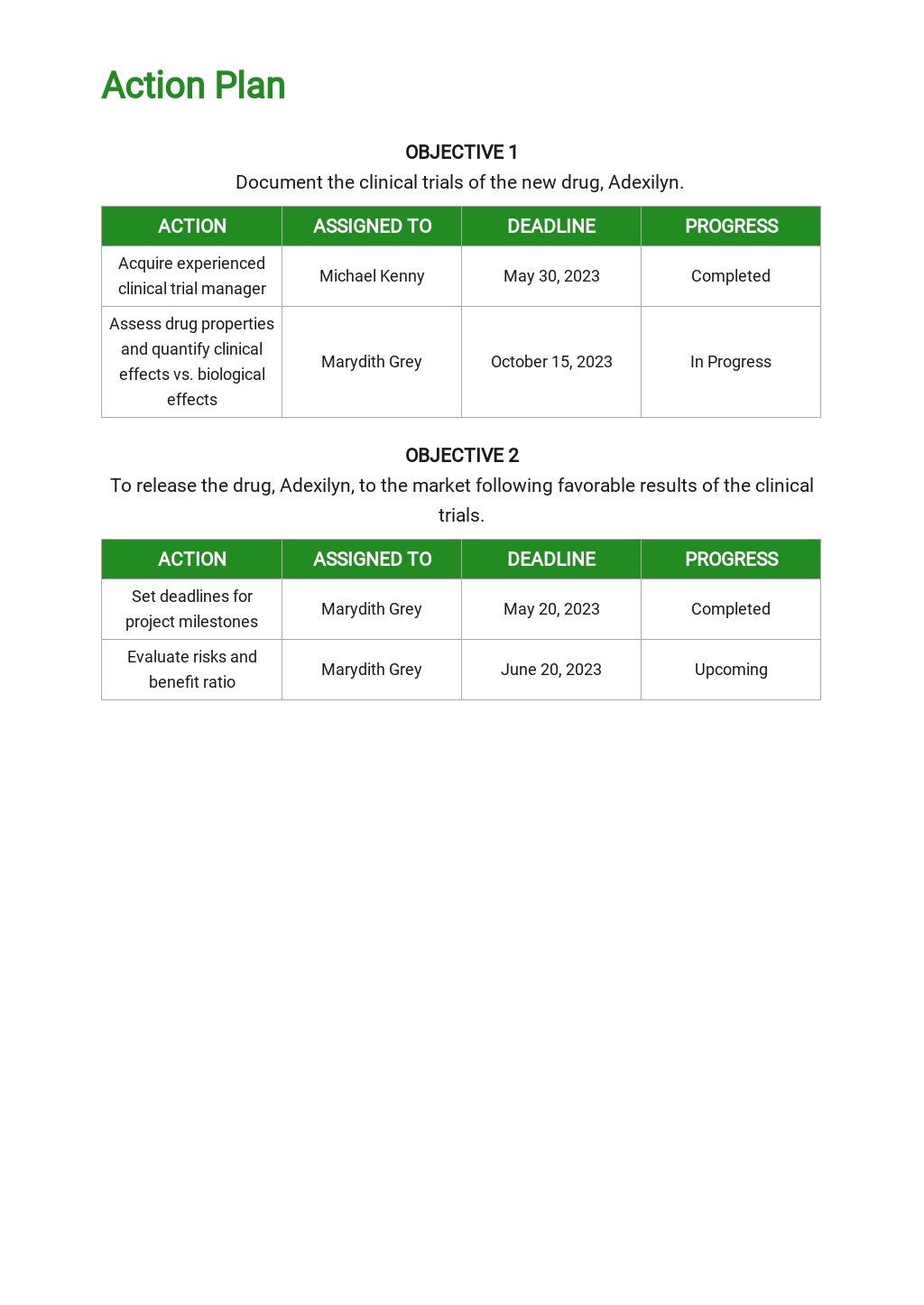
Clinical Development Plan Template - Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020. The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation.
Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020. The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation.
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
Clinical Development Plan Template
Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020. The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation.
Clinical Development Plan Template in Google Docs, Pages, Word
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
Clinical Development Plan Template Fda
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
Clinical Development Plan Template Peterainsworth
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
30 Clinical Development Plan Template Hamiltonplastering
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
Top 20 PowerPoint Templates to Create a Clinical Development Plan
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
Clinical Development Plan Template Google Docs, Word, Apple Pages
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
Top 20 PowerPoint Templates to Create a Clinical Development Plan
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
Clinical Development Plan Template Peterainsworth
The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.
The Clinical Development Plan (Cdp) Is A New Document That You Must Complete For The Eu Mdr Technical Documentation.
Learn how to plan and document your medical device's clinical evaluation according to the mdr and iso 14155:2020.